Gamma-Interferon

Wheelock first in the world proved that the human leukocytes are capable of synthesizing interferon.
Synthesis
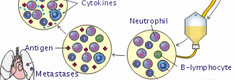
In human beings, the gene which codes for gamma-interferon (IFN-γ) is located in the 12-th chromosome. The cells which produce the endogen - gamma-interferon are: T-helpers- 0 and 1 types (CD4); cells with immunologic memory (CD45PA); T-killers (CD8); NK-cells; (CD16,CD56); dendrite cells (CD23,CD35) and B-lymphocytes (CD22, CD23).
Practically any antigens can cause the secretion of IFN-γ in one or other way. B-lymphocytes, for example, need intermediating agent so called interleukin-1 (IL-1) to produce IFN-γ. Where as Interleukin-2 (IL-2) induces the NK-cells to form IFN-γ. The human IFN-γ are the glycoproteins and have 2 forms. After the ribosomal synthesis of aminoacid chains forms a protein structure with molecular mass of 17000 Daltons which has two potential sites to form carbohydrate bonds. Then takes place so called the posttranslational glycolysis and forms glycoproteids of IFN-γ with molecular mass of 20000-25000 Daltons.
The recombinant IFN-γ, synthesized by bacteria or yeast do not go under glycolysis but their biochemical activities are similar to that of human IFN-γ as their activeness depends mainly on the protein molecules.
The pure drugs of IFN-γ are now available due to the recombinant technology. First the messenger RNA are extracted from the induced lymphocytes. Then with the help of a ferment (reverse transcriptase) DNA complements are formed which are incorporated with plasmids. With the help of these plasmids, the human genes responsible for IFN-γ productions are introduced into bacteria which inturn become the IFN-γ producers.
Recepters
The unique receptors to IFN-γ are located on the surface of most of the cells of organism but their expressions differ in different cells. Receptors with high affinity are located in the T- and B-lymphocytes, NK-cells, monocytes, macrophages, fibroblasts, neutrophils, endothelial and smooth muscle cells. For example, there are about 2500 IFN-γ recepters on the human fibroblasts.
So called the "bonding capacity constant" for recepters of IFN-γ is equal to 1-5 x 10-8 M.
Mode of action
During the secretion, IFN-γ influences on the secreting cells as well as on the cells around through IFN-γ - receptors. The first necessary step in the functioning of the γ-interferon is the interaction of IFN-γ with receptors located on the surface of the cells. IFN-γ can arouse defending or pathological effects. They induce the differentiation process of myoloid cells in bone marrow forming cells with highly affinated Fcg - receptors which combine with IgG-monomers. Where as in the matured granulocytes IFN-γ induces the expression of Fcg - receptors with medium affinity which combine with only the agregated IgG. IFN-γ also activates the antibody-dependent cytotoxins implemented by the matured granulocytes.
IFN-γ is an activator of macrophages and thus increases their antitumor activities. If the macrophages are infected by intracellular parasites, it activates macrophages which inturn destroy the parasites. Suppression of intracellular parasites under the influence of IFN-γ takes place in the nonmacrophage cells as well.
IFN-γ reinforces the antitumor activities of the cytotoxic lymphocytes. Together with lymphotoxins-CD4 or CD8, produced by lymphocytes, supress the tumor cell growth. The IFN-γ induces the expression of the receptors of lymphotoxins by acting in the nucleus of the target cells. IFN-γ increases the nonspecific activities of NK-cells.
IFN-γ is one of the factors which controls the differentiation of B-cells. It can either increase or decrease B-cell immune responses. In the late stages for example, IFN-γ increases the secretion of the immunoglobins.
IFN-γ plays an very important role in increasing the expression of HLA I and II class molecules on the cell membranes. More over, IFN-γ induces the expresion of HLA molecules of DR and DP, quicker than DQ. If the expression of HLA I and II class molecules on the pathological cells takes place more vigorously, then it becomes better recognised for the following destructive process. If this process takes place in the antigen-representing cells, then increases the formation of immune responses.
In case of viral infections, IFN-γ may cause considerable changes on the surface of cell-membrane which inhibits the adhesion and penetration of virus into the cells.
IFN-γ promotes the syntheses of ferment- oligoadenilat synthetases in cells. The polymers of oligoadenilat activates the endogen endonucleases which promotes the destruction of mRNA and rRNA, disturbing the intracellular synthesis in viral cells.
IFN-γ promotes the formation of ferment- proteinkinases resulting into the decrease of protein syntheses.
IFN-γ activates the osteoclasts increasing the resorption of bone-tissue.
Application
Note: 1 mcg of recombinant IFN-γ contains 104 IU (international unit).
The most effective dose of IFN-γ is 0.2 -10000 IU (0.02 ng-1 mcg) per 1 ml blood, depending on the type of cells against which it is used. IFN-γ is prescribed to treat the infectious, oncologic, autoimmune and allergic diseases.
3 schemes of injection IFN-γ are used: therapeutic, prophylactic and mixed. The appropriate dose of IFN-γ is calculated according to mass (kg) of the body or surface area (m2) of the body: 1 mcg is injected per kg mass or 40 mcg per m2 surface area of the body. IFN-γ may be injected subcutaneous, intracutaneous, intramuscular, intravenous, intranasal or even locally. After i.m. injection the peak concentration of IFN-γ in blood stream is observed through 4-6 hours. The half-life of IFN-γ is 7-9 hours but its effect in the body cells continues for 4 weeks. The maximum tolerable dose for i.m. may be up to 10 MIU (million international unit), where as incase of non-stop i.v. injection the maximum tolerable dose may be up to 100 MIU per day.
The therapeutic scheme is used for the treatment of infectious diseases- leishmania donovia, microbacterium lepra, taxoplasm gondii etc. IFN-γ is injected in doses 50-400 mcg per m2 surface area of the body, daily, for 10-30 days.
The scheme for prophylaxis is used for the prophylactic purposes to defend from the opportunist-infections in aids, for the prophylaxis of infective complications in chronic granulomatose (LGM) and in born T-cell immune deficiency. In these cases, IFN-γ is injected subcutaneous at the dose of 1.5 mcg per kg mass, from 3 times a week to once every 4 weeks, choosing the optimum number of injections.
The mixed scheme of injection of IFN-γ is used mainly in the treatment of oncological dieases. In the beginning, IFN-γ is injected everyday or every other day, hypodermic or i.m. or i.v., for 10-30 days. If it is possible to fetch the IFN-γ directly to the tumor, it would be the best method. Then we swich on to so called the maintaining dose, hypodermic or i.m. injection of IFN-γ once in every 1-4 weeks for 3-12 months.
It is not recommended to prescribe IFN-γ at the dose > 100 MIU, as it causes a dose-dependent fever (symptoms similar to cold) which is observed with in few hours after the hypodermic injection or after 30-60 minutes of i.v. injection. The repeated injection of IFN-γ results into anoreksia, weekness, malaise, sometimes with the symptoms of CNS disfunction (faintness, stuper, coma); chronic headache and orthostatic hypotension.
The over doses results into metabolic failure, in the forms of hyperkalimia, hypo- or hypercalcimia, slight increase in amiak and creatin levels. Also increases the levels of aspargin aminotransferases, γ-glutamiltranspeptidases and alkalic phosphotases. In some patiants may observe alopecia. The use of IFN-γ at the dose of < 1 MIU may result into transient lymphopenia and neutrophilic leucocytosis. The long time use of overdosed IFN-γ causes the reversible suppression of all the elements of bone marrow.
So as we see, the knowledge about the wide-range effects of γ- interferon allows to utilize it rationally during the treatment and prophylaxis of several diseases.